Home
Home
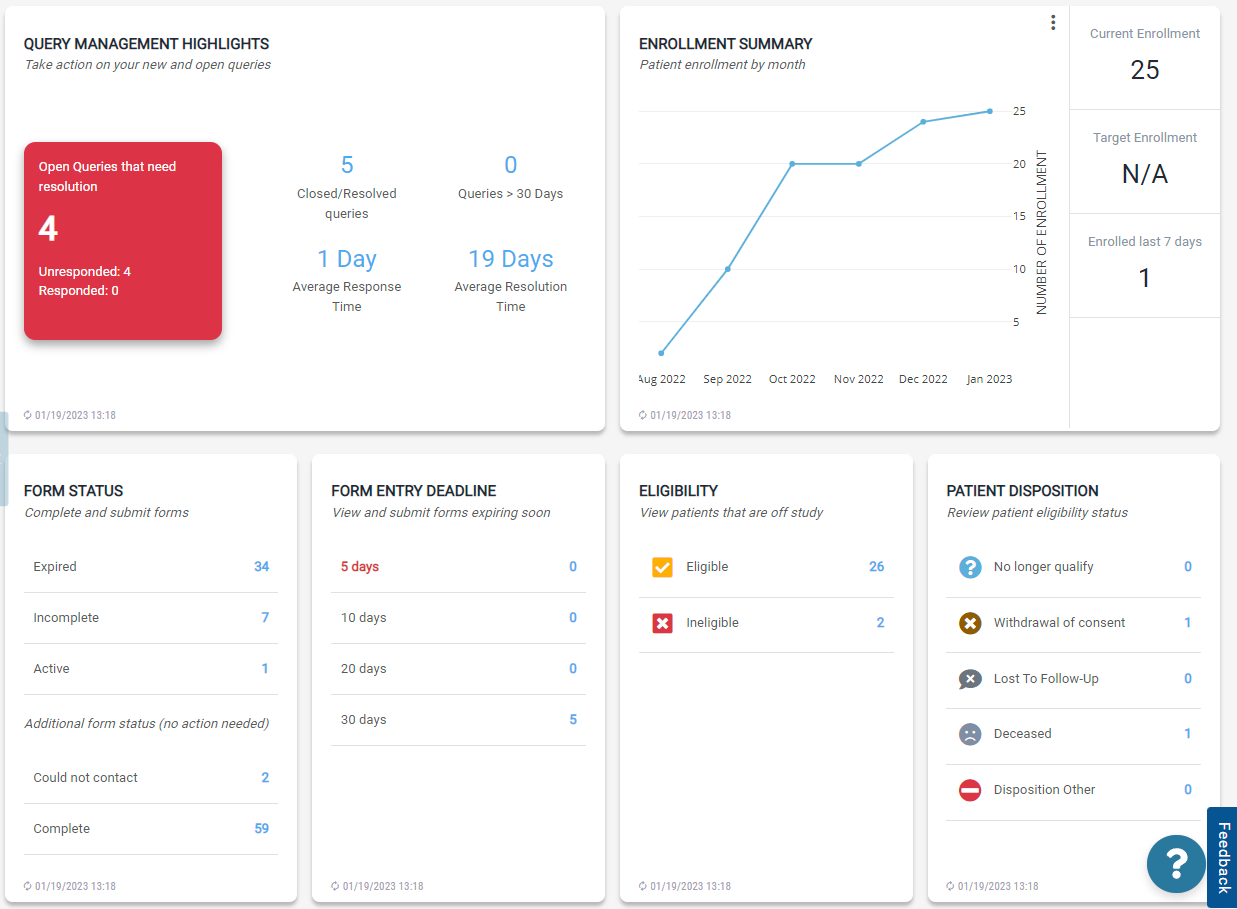
After logging in, the user is taken to the Talosix home screen which is personalized to the user's role in the study. The user can navigate through different screens within the tool using the left-hand side navigation. The main area of the screen is a dashboard with modules displaying pertinent information for the user to review and take action. These modules include:
- Querry Management Highlights
- Enrollment Summary
- Form Status
- Form Entry Deadline
- Eligibility
- Patient Disposition
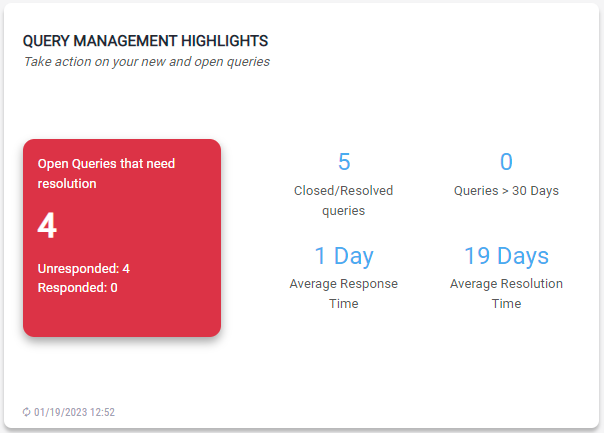
Querry Management Highlights
The module provides a summary of new and open queries as well as the average response time and average resolution time. To view the patient records corresponding to the highlighted items:
- Click on the red square to view the open queries that need resolution.
- Click on the link to view closed/resolved queries.
- Click on the link to view queries greater than 30 days.
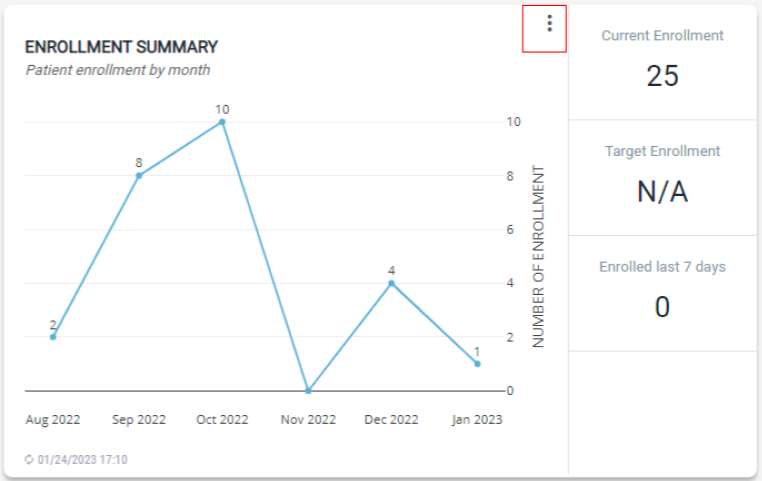
Enrollment Summary
The module provides a summary of patient enrollment, the current enrollment, target enrollment, and enrolled last 7 days.
- To view cumulative enrollment, click on the vertical menu and select Cumulative Sum. This is the total number of patients enrolled up to that month.
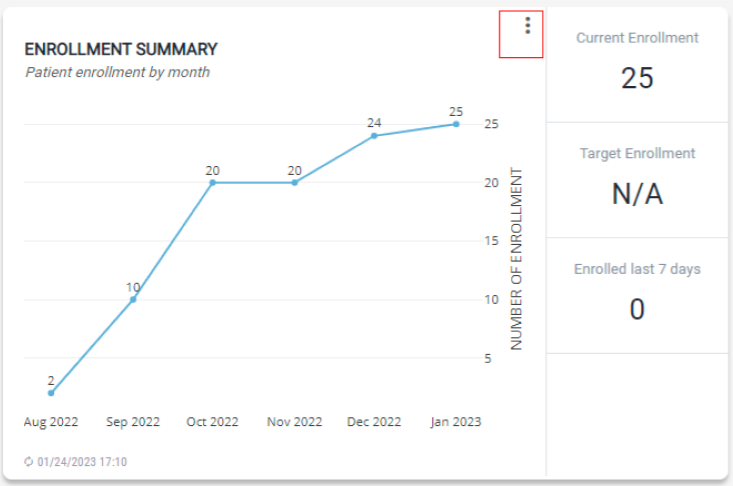
- To view the number of patients by month, click on the vertical menu bar and select Show by Month.
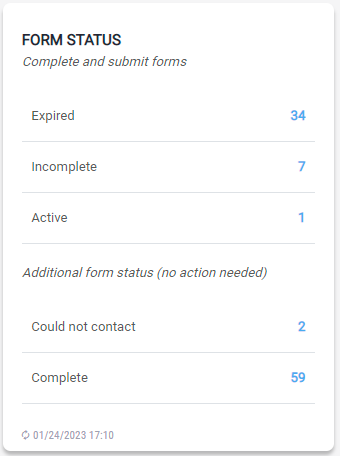
Form Status
The module provides a high-level view of the forms in different states. The user can select a status to view those forms and take action.
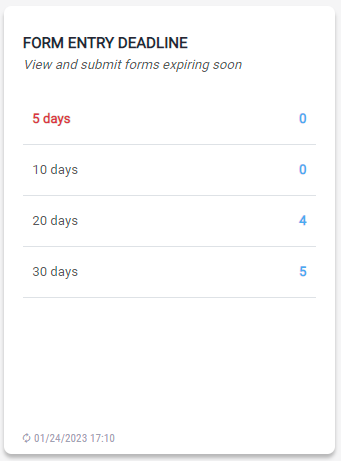
Form Entry Deadline
The module provides a timeline of forms expiring soon. The user can quickly access the forms that are due within 5 days by clicking on the numeric link to complete those forms.
Eligibility
The module provides a summary view of the total number of patients who are eligible and those who are off-study. The user can click on the links to view the records.

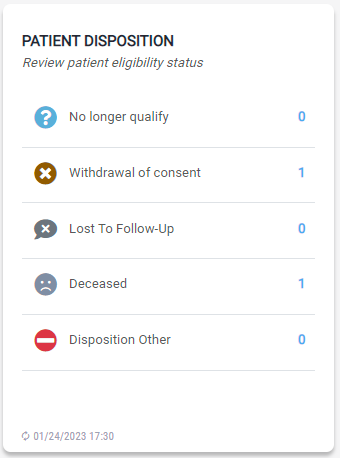
Patient Disposition
The module provides a summary of all patient statuses in the study. This includes information such as whether the patient withdrew from the trial due to an adverse event or other reasons.